Metals are some of the most common contaminants found in soil and groundwater and can be some of the more challenging constituents to remediate. Treatment and cleanup methods are often invasive — pump-and-treat, for example, requires the installation of a network of extraction wells and the construction and operation of a facility to treat contaminated groundwater. Soil contamination is often excavated and disposed of in large volumes at hazardous waste disposal facilities. Even passive in situ remediation of metals-impacted groundwater and soil can be costly and may require multiple rounds of treatment to achieve the required results.
Phytoremediation offers a sustainable approach that can degrade, remove or immobilize contaminants while minimizing cost and disruption to a site. Phytoremediation is a novel approach to reducing or immobilizing metals and other inorganic contaminants, one that provides a greater net environmental benefit. Phytoremediation strategies have been studied and experimentally trialed for a wide variety of metals, including arsenic, cadmium, cobalt, copper, lead, zinc, uranium and many others.
Phytoremediation refers to a wide variety of treatment approaches that are facilitated by certain plant species. Two of the most common phytoremediation technologies are phytoextraction and rhizofiltration. Some plants are capable of taking up dissolved contaminants with groundwater, a mechanism known as phytoextraction. Other plant species have root networks that can create the conditions needed to reduce contaminant mobility by sequestering aqueous contaminants in the sorbed phase. This process is known as rhizofiltration.
Decades of industrial activities, including laboratory research, mining, nuclear fuel processing, and nuclear weapons manufacturing and testing, have resulted in the accumulation of radioactive uranium in soil and groundwater at numerous sites across the U.S. Uranium is semi-soluble and mobile in the subsurface leading to the formation of groundwater contamination plumes at many sites.
Both phytoextraction and rhizofiltration are promising mechanisms that can be used to reduce the concentration and/or mobility of uranium and other metals in contaminated soil and groundwater. The implementation and effectiveness of a phytoremediation mechanism is driven by plant species, climate, and site-specific soil and contaminant characteristics. This paper is focused on the use of phytoextraction and rhizofiltration for uranium remediation. The majority of uranium-focused phytoremediation studies have been small-scale experiments conducted in laboratories and at field pilot test sites. Based on the progress of phytoremediation technologies overall, it may be time to give greater consideration to phytoremediation for uranium cleanup.
White Paper
Using Plants for Sustainable, Effective Remediation of Groundwater and Soil
BY Emily Pulcher, PE
Metal contamination in soil and groundwater has resulted from a variety of industrial activities. Traditional remediation approaches, such as pump-and-treat or soil excavation, can be energy-, cost-, and carbon-intensive. Phytoremediation can offer a sustainable alternative to these approaches by reducing contaminant concentration or mobility while providing a greater net environmental benefit.

Metals are some of the most common contaminants found in soil and groundwater and can be some of the more challenging constituents to remediate. Treatment and cleanup methods are often invasive — pump-and-treat, for example, requires the installation of a network of extraction wells and the construction and operation of a facility to treat contaminated groundwater. Soil contamination is often excavated and disposed of in large volumes at hazardous waste disposal facilities. Even passive in situ remediation of metals-impacted groundwater and soil can be costly and may require multiple rounds of treatment to achieve the required results.
Phytoremediation offers a sustainable approach that can degrade, remove or immobilize contaminants while minimizing cost and disruption to a site. Phytoremediation is a novel approach to reducing or immobilizing metals and other inorganic contaminants, one that provides a greater net environmental benefit. Phytoremediation strategies have been studied and experimentally trialed for a wide variety of metals, including arsenic, cadmium, cobalt, copper, lead, zinc, uranium and many others.
Phytoextraction
The uptake of a dissolved contaminant and subsequent storage in a plant’s biomass via phytoextraction renders a contaminant immobile. Uranium complexes enter the plant and pass through the selective root membranes. From inside the root, the contaminant is transported to the aboveground plant biomass (shoots) through the xylem, where it then resides in the stems and leaves of the plant.
Depending on the plant’s survival mechanisms and efficiency at transporting specific uranium complexes through the plant transport network, phytoextraction may result in the significant accumulation of uranium in the roots or aboveground shoots, stems and leaves of a plant. As stated in a study published in Environmental Science and Pollution Research, plants used for phytoextraction are usually hyperaccumulators, which survive by storing large concentrations of metals in their stems and leaves and smaller concentrations in their roots. This is a minimally invasive approach to remediation that preserves both soil structure and fertility, while reducing contaminant mass in soil and groundwater.
The ability of a plant to store large concentrations of contaminants in its aboveground plant biomass is quantified by the bioaccumulation coefficient (BC). The BC is defined as the ratio of uranium concentration in aboveground plant biomass to the uranium concentration in soil. This value varies greatly across plant species and the BC is affected by several factors, including soil and plant characteristics and growing conditions. Soil characteristics impacting BC include the presence of chelating agents that facilitate complexation with the radionuclide and the presence of other ions and nutrients. Soil pH and porosity are also important to plant health and therefore affect the BC. Plant characteristics impacting BC include the ability of the plant to internally transport the radionuclide from roots to shoots and the ability to survive high concentrations of the radionuclide. Another desirable characteristic for plants used in phytoextraction is the ability to quickly grow a large amount of biomass, to allow for greater removal of the radionuclide from the subsurface.
Some of the primary challenges of efficient uranium phytoextraction are the bioavailability and solubility of uranium in the soil and groundwater. Soil amendments can be used to improve the uptake of uranium by plants and typically include organic acids that lower the soil pH and increase the solubility of uranium complexes. According to a study published in Current Opinion in Biotechnology, the addition of citric acid to uranium-contaminated soil used to grow a variety of plant species resulted in significantly increased shoot uranium concentration as compared to unamended soil. The results of this study are illustrated in Figure 1.
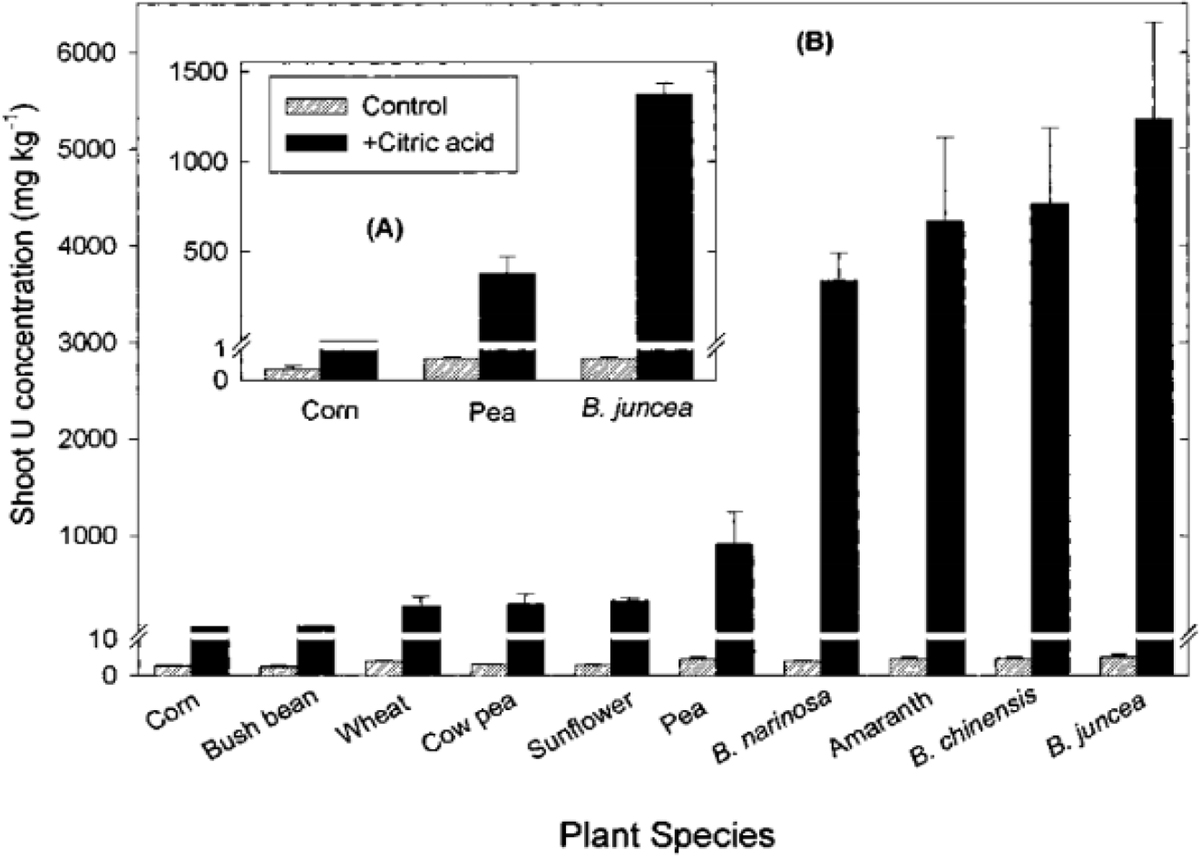
Figure 1: Effect on uranium concentration in shoots of several plant species from the addition of citric acid (20 mmol per kg soil) to two uranium-contaminated soils. One soil had a uranium concentration of 280 mg/kg (A), and another had a uranium concentration of 750 mg/kg (B). Huang, J. W., Blaylock, M. J., Kapulnik, Y., & Ensley, B. D., 1998.
The study also showed that chelating agents aid in the internal transport of uranium through the plant. Chelating agents may occur naturally, or synthetic chelating agents may be introduced to the soil. Ethylenediaminetetraacetic acid (EDTA) is a common synthetic chelator used to enhance root-to-shoot transfer by desorbing metals from the soil matrix to a loosely bound form more easily transported through the plant structure. However, careful consideration should be given to the use of soil amendments to increase the solubility and bioavailability of uranium since they may result in the unintended migration of contaminants in the subsurface. The risks and benefits of soil amendments should be carefully considered prior to use.
A plant’s ability to survive high concentrations of toxic metals is also a limiting factor in phytoextraction efficacy. One strategy to increase plants resistance to toxic metals is the use of genetically modified bacteria both in planta and in the surrounding soils. According to a study published by the National Library of Medicine, bacteria may be genetically modified to house metal sequestration systems designed to reduce phytotoxicity and protect the plants’ metabolic pathways from harmful metal concentrations.
Another challenge in the phytoextraction of uranium and other contaminants is controlling the final fate of the contaminant in the environment. One study showed Scotch pine (Pinus sylvestris L.) demonstrated efficient uptake of uranium and storage at high concentrations within the plant foliage. However, this foliage is shed from the tree and an estimated 97% of the accumulated uranium is returned to the soil.
For this and other reasons, the final fate of plant biomass must be considered when selecting plant species for phytoextraction to prevent reintroduction of the contaminant to the environment or food chain. Appropriate disposal methods for plants that have accumulated the target contaminant must be developed prior to technology implementation. Though unlikely, the potential phytoaccumulation of uranium or other fissile radionuclides at high concentrations may lead to additional regulatory requirements related to crop management. The most likely potential regulatory impact would be a requirement to dispose of harvested crops as hazardous waste. Reducing the overall volume of biomass prior to final disposal may be necessary to maintain cost-effectiveness. Overall waste volume reduction methods primarily include composting, drying and incineration. Disposal methods for harvested plants containing concentrations of uranium or other metals exceeding regulatory limits will likely require transportation to an appropriate waste disposal facility.
Rhizofiltration
Rhizofiltration complements phytoextraction by sequestering target contaminants in the plant root zone. In contrast to phytoextraction, plants use rhizofiltration to survive by specifically excluding uranium complexes from passing through root membranes. As a result, the radionuclides accumulate in the rhizosphere, or the area surrounding the root zone of the plant. The radionuclides are not removed from the subsurface but are instead immobilized to prevent transport, thus mitigating exposure pathways. They may adsorb to the root surface or form static complexes with plant exudates. The efficiency of rhizofiltration is dependent on pH, temperature and ionic populations.
Symbiotic fungi present in the root zone can also positively impact the stabilization of radionuclides by forming static complexes or providing additional surface area for sorption. Arbuscular mycorrhizal fungi (AMF) are common symbiotic fungi that improve the efficiency of rhizofiltration by increasing the surface area available for adsorption and providing a source of molecules to form complexes with uranium. Shown in Figure 2, AMF can grow naturally in the root zone. However, as discussed by Sharma, Singh and Manchanda, AMF may also be intentionally introduced to the soil as inoculum to help plants survive and grow by protecting them from fatal levels of toxicity at the root.

Figure 2: AMF growth (white) on plant root structure.
As described above, rhizofiltration can be implemented in situ to immobilize contaminants in soil and groundwater; however, it can also be applied with water plant species employed in a flow-through pond series configuration to achieve contaminant removal, as illustrated in Figure 3. Terrestrial plants may also be used for rhizofiltration of contaminated aqueous streams. Dushenkov has shown that sunflowers have been proven effective in rhizofiltration and a uranium removal of 95% from the contaminated stream was observed in a laboratory setting, with BC values as high as 30,000.
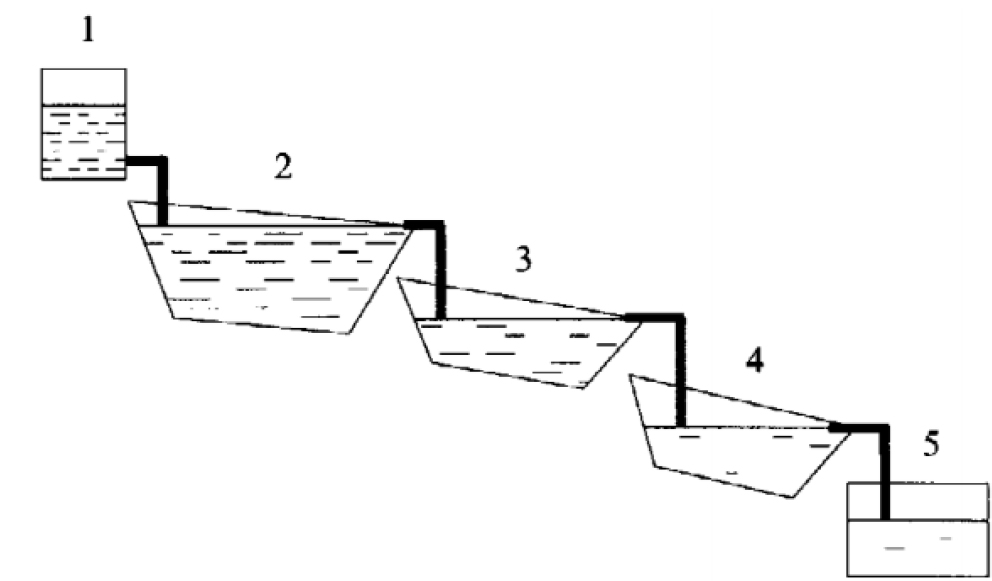
Figure 3: Schematic of a series configuration of ponds used for rhizofiltration. Dashed line hatching represents radionuclide concentration. S. Dushenkov, 2003.
One of the most significant challenges associated with rhizofiltration is the small area of influence of the root zone. Radionuclide stabilization within and in the immediate vicinity of the rhizosphere may be efficient, but the volume of soil influenced by the rhizosphere is very small. It is difficult to immobilize significant contaminant mass when only treating a small fraction of the contaminated media. A published study states that dense plant growth to minimize the space between rhizospheres is necessary for impactful rhizofiltration.
Rhizofiltration has been most widely studied in ex situ applications, primarily as a water treatment method, making the approach a promising candidate for treating runoff from mining operations and preventing contamination. However, rhizofiltration may also be used as the ex situ treatment component in a pump-and-treat-style groundwater remediation system. Treated water can then be reintroduced to the aquifer to preserve the resource. Similar to plants used in phytoextraction, appropriate disposal techniques must be considered if harvested plants’ root surfaces accumulate contaminants at levels exceeding regulatory thresholds.
Conclusions
Phytoextraction and rhizofiltration are two potential approaches to sustainably remediate soil and groundwater contaminated with uranium and other metals. When soils are amended with organic acids and synthetic chelators, the bioavailability of uranium increases significantly. However, the use of such amendments to increase bioavailability also carries the risk of furthering contaminant migration if sufficient capture is not achieved.
With soil amendments, several plant species have been shown to hyperaccumulate uranium in shoots at levels high enough to warrant further pilot testing. Rhizofiltration is a promising approach to treating aqueous waste streams or groundwater containing uranium, with species such as sunflowers demonstrating a BC of up to 30,000.
The continued pursuit of phytoremediation technologies to address uranium and other metals contamination is important to effectively and sustainably address today’s environmental contamination challenges. A variety of plant species and soil amendments have demonstrated effective uptake of radionuclides from media in laboratory and small-scale field applications. Phytoremediation approaches are environmentally attractive, sustainable, and offer long-term solutions for persistent contaminants. Modern pilot tests, conducted in a variety of field applications, and the refinement of plant disposal methods are key to advancing the development and implementation of phytoremediation technologies on a larger scale.
✖
READ White Paper
✖
