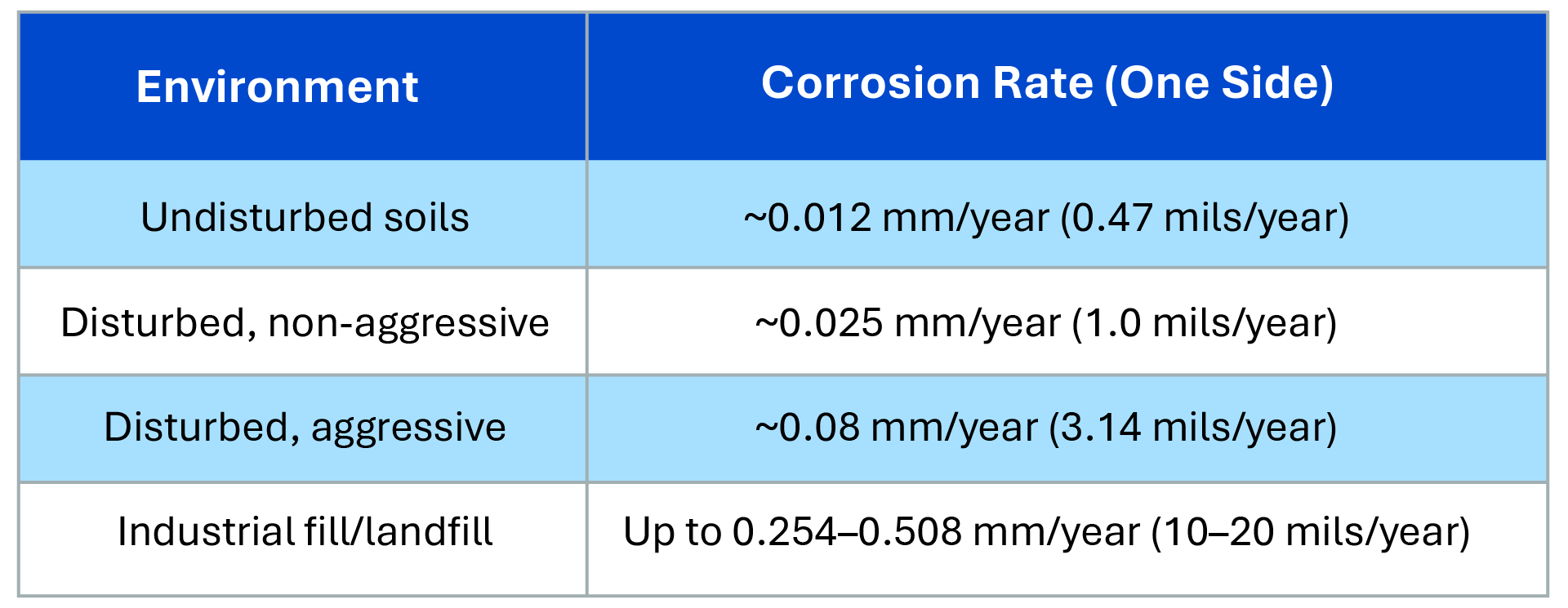
Metallic corrosion in the United States has resulted in direct costs of $276 billion in a single year, including $137.9 billion throughout infrastructure, according to a study by the U.S. Federal Highway Administration. Those totals include direct costs of $6.9 billion for electric utilities, with corrosion damaging foundations for transmission lines, substations and other infrastructure.
Transmission and distribution industry professionals cite corrosion risks as a leading reason why they are hesitant to use helical piles and embedded structures in their foundations. Concerns about long-term durability in corrosive environments can make these options seem less appealing.
Despite such concerns, helical piles can still be a sound foundation choice for substations and other structures where high water tables, collapsing soils or other unfavorable conditions are present. Requiring no curing time, helical piles offer the advantage of immediate load-bearing capability upon installation. Additionally, their screw-like design allows for quick and efficient installation, particularly in confined spaces or near existing structures.
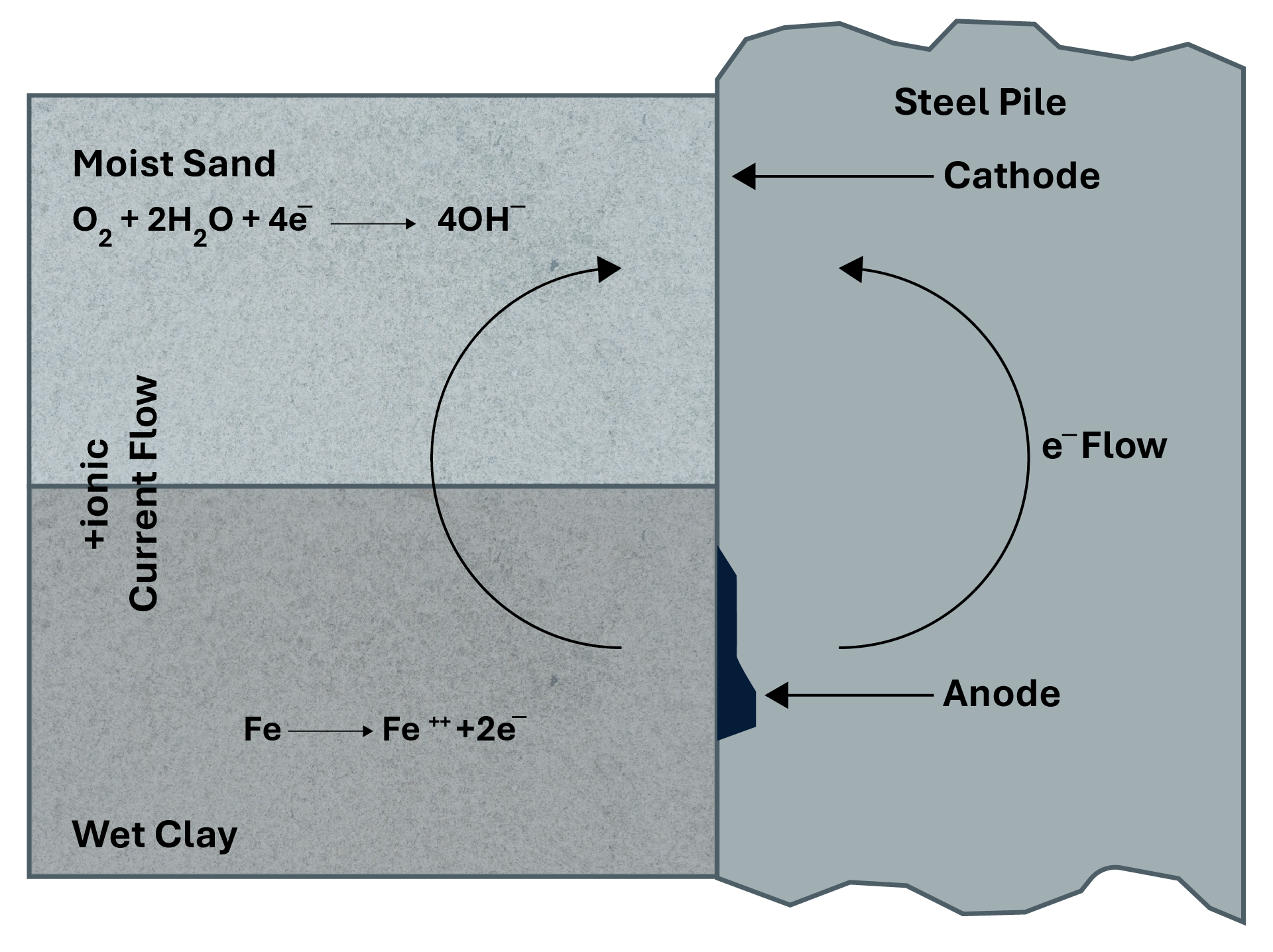
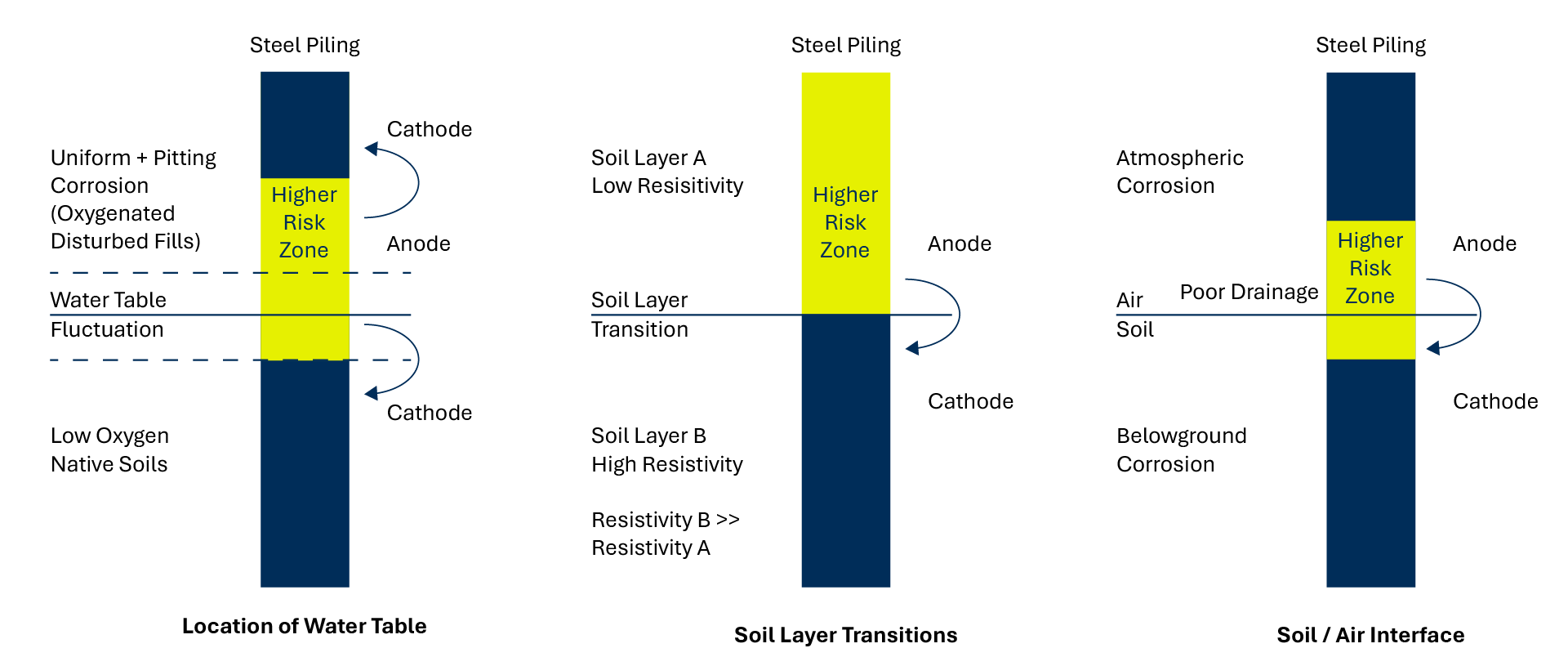
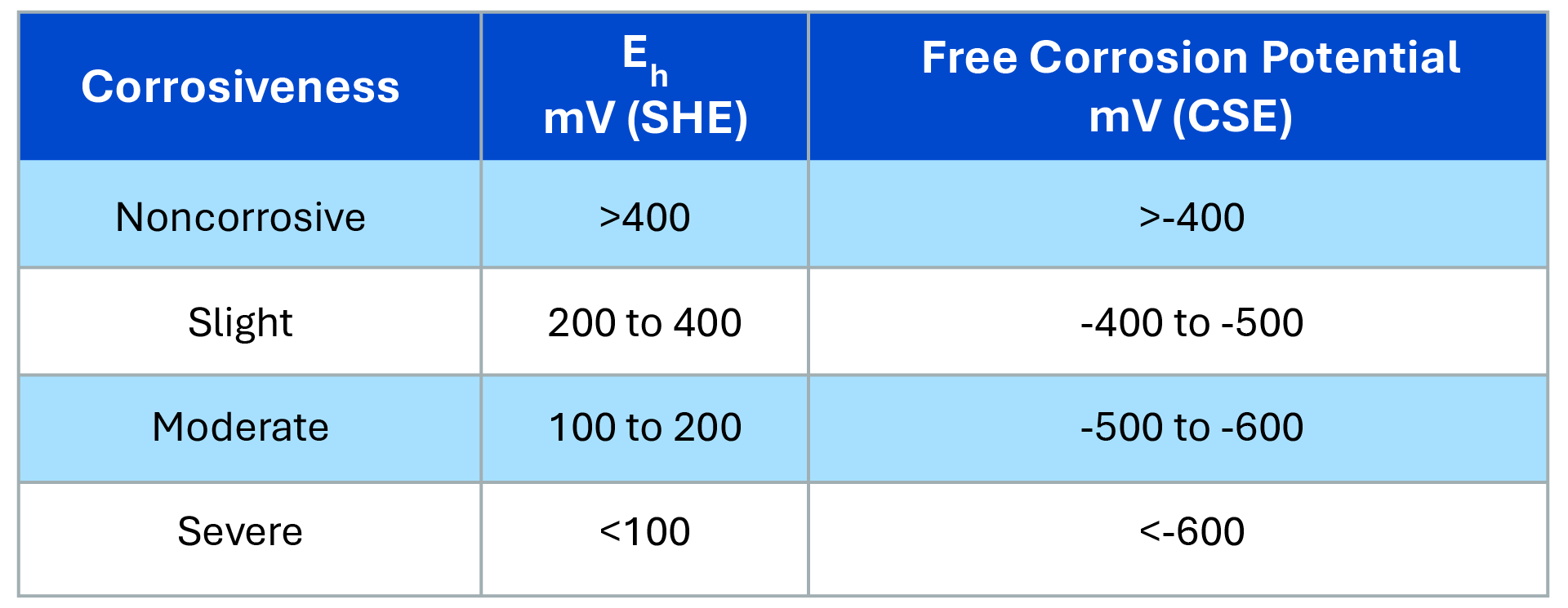
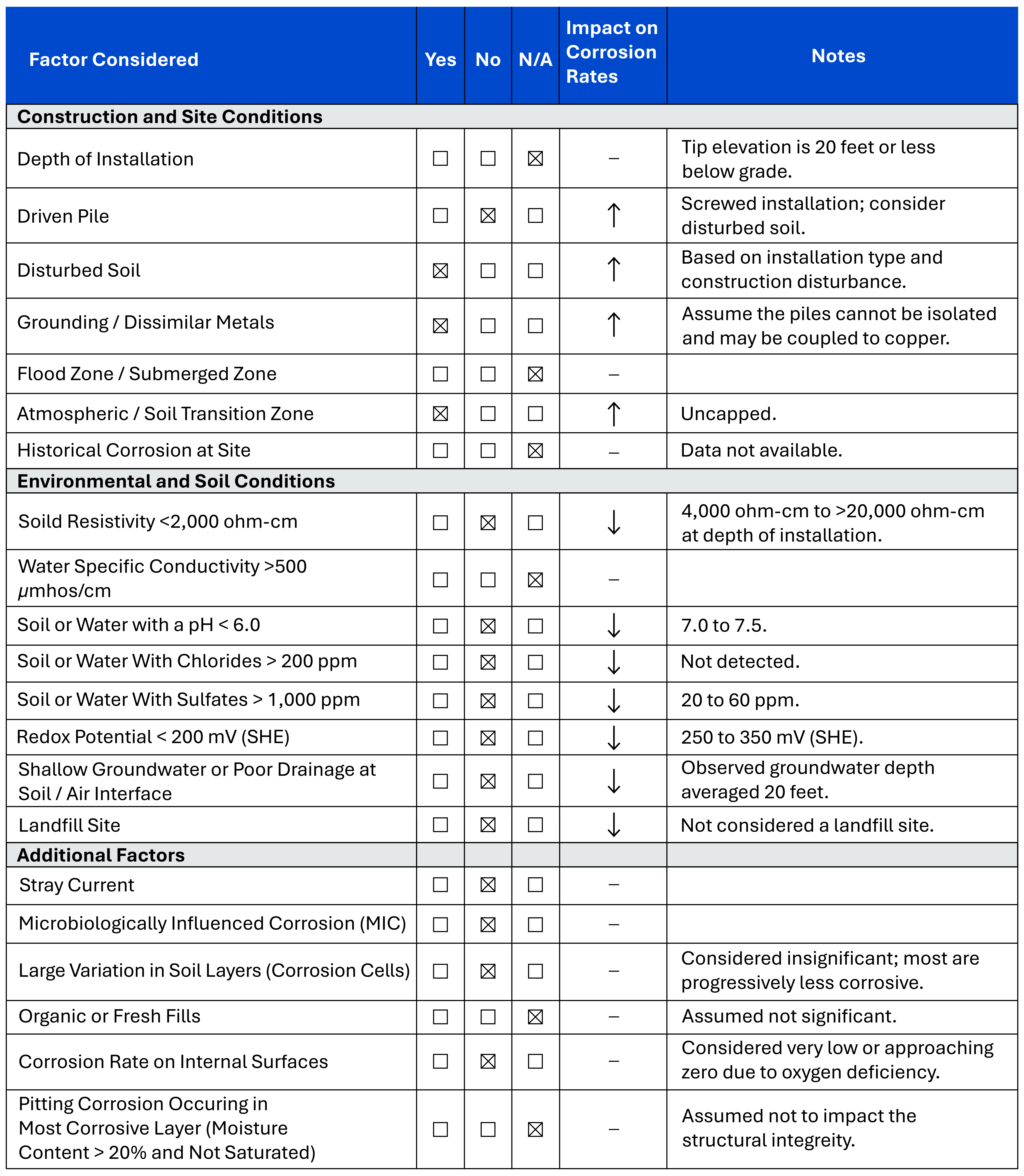
Although both helical piles and embedded structures can be susceptible to corrosion, the application of protective technologies effectively mitigates these risks. With the use of advanced protective coatings, cathodic protection and other solutions, both can be designed to withstand corrosive conditions, extending their lifespan and significantly reducing the cost of maintenance and replacement.